Areas of Study
Vaccine development for bacterial and viral pathogens
Education
- Jadavpur University, Kolkata, India2021PhD
Research
APC-targeting approach for adjuvant-free mucosal vaccination. Research in our laboratory is focused on developing vaccines and vaccine platforms for mucosal vaccination for pathogenic microbes that cause significant public health burden. Immunization via mucosal route evokes a robust mucosal immunity, however, currently mucosal vaccine technologies that ensure high safety standards while enabling adequate efficacy are very limited. We utilize an antigen-presenting-cell (APC)- targeting approach to enhance immunogenicity of antigens, thereby eliminating the need for adjuvants which oftentimes cause excessive inflammation. Since our technology is directed to the human FcγRI, its immunomodulatory effect is focused on dendritic cells, monocytes and macrophages that predominantly express this receptor. In contrast, most adjuvants stimulate a diverse cell types, thereby causing bystander immune activation. We have demonstrated the efficacy of this adjuvant-free mucosal (intranasal) vaccine platform in a mouse model of pneumococcal pneumonia.
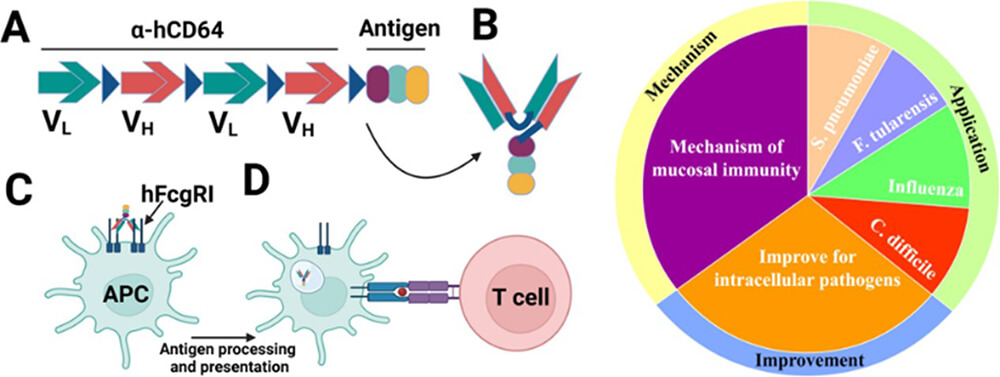
Vaccine development against the Streptococcus pneumoniae. Streptococcus pneumoniae (Sp) is a common bacterial pathogen that poses a significant public health burden globally. In 2016, it was responsible for over 197 million infections and 1.2 million deaths, making it a leading cause of lower respiratory tract infections. While currently available polysaccharide conjugate vaccines are effective, it does not cover an ever increasing number of antigenically distinct Sp strains that escape vaccine mediated immunity. Moreover, promoting immunity at the site of colonization that is nasopharyngeal mucosa may has added benefit of rendering the infection noninvasive as well as reducing community spread by person-to-person contact. Thus, we are applying our APC-targeted vaccine approach to develop an intranasal vaccine against Sp.
Vaccine development against the bio-threat agent Francisella tularensis. F. tularensis is a highly virulent category A pathogen and is considered a potential bioweapon. Currently there is no licensed vaccine for general population, however, a live vaccine strain of F. tularensis (Ft-LVS) was used for protection of laboratory personals. This strain is also used widely for investigation of novel vaccine candidates against tularemia. We are currently investigating the APC targeted vaccine approach to develop mucosal (intranasal) vaccine against tularemia.
Development of a novel vaccine platform based on an autotransporter system and attenuated EHEC. Clostridium difficile infection (CDI) can range in severity from asymptomatic to severe and life-threatening, especially among the elderly. People are most often infected in hospitals, nursing homes, or other medical institutions, although CDI in the outpatient setting is increasing. Since 1996 the incidence of CDI has more than doubled resulting in up to three million cases each year in the United States and making CDI the most common bacterial cause of diarrhea in the United States. We are currently investigating the APC targeted vaccine approach to develop mucosal vaccine against CDAD.
Investigation of the mechanism and improvement of the APC-targeted vaccine approach: In addition to the application, we are currently investigating mechanism of the mucosal immunity elicited by the APC-targeted vaccine approach. Moreover, we are also devising novel approaches to further improve the efficacy of the APC-targeted vaccine approach for intracellular pathogens.
Publications
Majumder S, Li P, Das S, Nafiz TN, Kumar S, Bai G, Dellario H, Sui H, Guan Z, Curtiss R 3rd, Furuya Y, Sun W. A bacterial vesicle-based pneumococcal vaccine against influenza-mediated secondary Streptococcus pneumoniae pulmonary infection. Mucosal Immunol. 2024 Apr;17(2):169-181. doi: 10.1016/j.mucimm.2024.01.002. Epub 2024 Jan 11. PubMed PMID: 38215909; PubMed Central PMCID: PMC11033695.
Sunagar R, Singh A, Kumar S. SARS-CoV-2: Immunity, Challenges with Current Vaccines, and a Novel Perspective on Mucosal Vaccines. Vaccines (Basel). 2023 Apr 15;11(4). doi: 10.3390/vaccines11040849. Review. PubMed PMID: 37112761; PubMed Central PMCID: PMC10143972.
Shoudy LE, Namjoshi P, Giordano G, Kumar S, Bowling JD, Gelhaus C, Barry EM, Hazlett AJ, Hazlett BA, Cooper KL, Pittman PR, Reed DS, Hazlett KRO. The O-Ag Antibody Response to Francisella Is Distinct in Rodents and Higher Animals and Can Serve as a Correlate of Protection. Pathogens. 2021 Dec 20;10(12). doi: 10.3390/pathogens10121646. PubMed PMID: 34959601; PubMed Central PMCID: PMC8704338.
Holland-Tummillo KM, Shoudy LE, Steiner D, Kumar S, Rosa SJ, Namjoshi P, Singh A, Sellati TJ, Gosselin EJ, Hazlett KR. Autotransporter-Mediated Display of Complement Receptor Ligands by Gram-Negative Bacteria Increases Antibody Responses and Limits Disease Severity.
PubMed Bibliography:
View Sudeep Kumar's bibliography on the National Institute of Health's PubMed website.