Areas of Study
Cancer, cell migration, and the cytoskeleton
Education
- National Institutes of Health2011-2016Post-Doc
- University of Washington2011PhD
- University of Oregon2004BS
Research
Cell migration is a fundamental process crucial for various physiological events, including embryonic development, immune surveillance, and wound healing. However, the deregulated migration of tumor cells is a hallmark of cancer metastasis, enabling them to spread and colonize vital organs, which is the primary cause of cancer related deaths. The Logue Lab is dedicated to understanding the molecular mechanism(s) by which cells migrate within the intricate and often confining microenvironment of tissues. We are particularly interested in the phenomenon where cancer cells undergo a phenotypic transition from mesenchymal to amoeboid (i.e., bleb-based) migration in response to mechanical confinement and low adhesion. Given that cancer cells frequently encounter confined spaces within tissues and that the phenotypic transition can be induced by certain drug treatments (e.g., Src family kinase inhibitors), amoeboid migration likely plays a critical role in promoting distant metastasis across diverse mechanochemical environments.
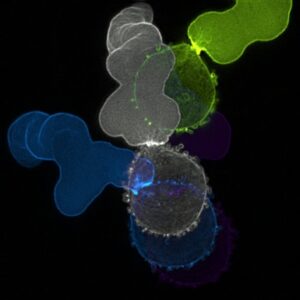 At left, temporal color-code image of F-actin in a melanoma cell undergoing fast amoeboid (leader bleb-based) migration.
At left, temporal color-code image of F-actin in a melanoma cell undergoing fast amoeboid (leader bleb-based) migration.
To comprehensively study amoeboid migration, our research combines in vitro assays that allow for precise control over cell confinement and adhesivity (e.g., micropatterned surfaces) with high-resolution light microscopic imaging. We utilize tools like genetically encoded fluorescent biosensors to dissect the molecular and biophysical mechanisms underlying confined cell migration. Our goal broadens beyond cancer cells to encompass understanding how diverse motile cell types, including immune cells, adapt their migration strategies to the various mechanochemical properties of tissues. Ultimately, by elucidating the molecular mechanism(s) governing amoeboid (i.e., bleb-based) migration, we aim to rationally develop novel therapeutics that specifically prevent or abate the growth and migration of cancer cells, thereby addressing the significant clinical challenge of metastasis. This is particularly important as cancer cells show remarkable plasticity in their migration modes, which can lead to resistance against therapies targeting a single mechanism.
Publications
Caruso AP & Logue JS (2024). The biophysics of cell motility through mechanochemically challenging environments. Current Opinion in Cell Biology (PMID: 39053178)
Ullo MF & Logue JS (2021). ADF and cofilin-1 collaborate to promote cortical actin flow and the leader bleb-based migration of confined cells. eLife (PMID:34169836)
View Jeremy S. Logue's articles on the National Institute of Health's PubMed website.
